Therefore, by dividing the ACR value by the resistance of the current-sense resistor RSNS, the battery capacity in Ah (Ah) is obtained. Both the ADC conversion result and the accumulated result are signed. According to the connection method in Figure 1, CR is positive and ACR is increasing during charging; CR is negative and ACR is decreasing during discharging. The external microcontroller can read the CR and ACR values ​​and convert them to the actual charge and discharge current and charge value. N-Ethylpyridinium Chloride,Cas 2294-38-4,N-Ethylpyridinium Chloride 2294-38-4,Pyridine Ionic Liquids Henan Tianfu Chemical Co.,Ltd , https://www.hnionicliquids.com
Lead-acid batteries have large capacity and low internal resistance (generally 400Ah 2V battery internal resistance is about 0.5mΩ), which can be used for large current discharge, but it is bulky and bulky, and is not easy to carry. It is commonly used in automotive and industrial applications. Its electrode material contains lead, which can cause great pollution to the environment. Lead-acid batteries have low requirements for charging control and can be floated.
Nickel-cadmium battery has large capacity, low internal resistance and stable discharge voltage, and is suitable for DC power supply. Compared with other types of batteries, nickel-cadmium batteries are resistant to overcharging and overdischarging, and are easy to operate, but have a memory effect and should be charged after full discharge. The electrode material contains highly toxic heavy metal cadmium, and its market share is getting smaller as environmental protection requirements increase. 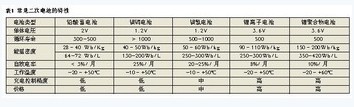
Nickel-metal hydride batteries are developed on the basis of nickel-cadmium batteries. Metallic hydrogen is used instead of toxic cadmium, which can replace nickel-cadmium batteries in most occasions. Its capacity is about 1.5 to 2 times that of nickel-cadmium batteries, and there is no memory effect. Compared with nickel-metal hydride batteries, it has higher requirements for charging control and is currently used in a large number of portable electronic products.
Lithium-ion batteries are the most common secondary lithium batteries at present, and have high energy density. Compared with high-capacity nickel-cadmium/nickel-hydrogen batteries, their energy density is 1.5 to 2 times that of the former. Its average operating voltage is 3.6V, which is three times that of nickel-cadmium batteries and nickel-hydrogen batteries. It has a large internal resistance, cannot handle large currents and discharges, and requires precise charge and discharge control to prevent battery damage and achieve optimum performance. Lithium-ion batteries are widely used in a variety of portable electronic products, including mobile phones, notebook computers, mp3 and so on.
Lithium polymer battery is a new type of secondary lithium battery with larger capacity; lower internal resistance, allowing 10C charge and discharge current. It requires precise charge and discharge control like a lithium-ion battery. At present, lithium polymer batteries are mainly used in applications that require large current charge and discharge, such as power/model cars. Rechargeable battery capacity estimation method.
In most portable applications, you need to know the remaining battery capacity to estimate battery life. 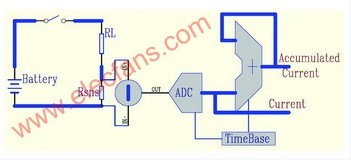
Figure 1 simplified battery fuel gauge block diagram
The earliest method of application was to obtain the remaining capacity by monitoring the open circuit voltage of the battery. This is because there is a certain relationship between the battery terminal voltage and the remaining capacity, and the remaining capacity can be estimated by measuring the battery terminal voltage. The limitations of this method are: 1) The relationship between open circuit voltage and capacity is different for batteries produced by different manufacturers. 2) Relatively accurate results can only be obtained by measuring the open circuit voltage when the battery is unloaded. However, most applications need to know the remaining capacity of the battery during operation. At this time, the voltage drop generated by the internal current of the load current will affect the open circuit. Voltage measurement accuracy. The dispersion of the internal resistance of the battery is large, and the dispersion becomes larger as the battery ages, so it is very difficult to compensate for the error caused by the voltage drop. In summary, the method of estimating the remaining capacity of the battery in real time by the open circuit voltage cannot achieve sufficient accuracy in practical applications, and can only provide a rough reference value.
Another method that is heavily used is to estimate the remaining battery capacity by measuring the net charge flowing into/out of the battery. This method integrates the total current flowing into/out of the battery, and the resulting net charge is the remaining capacity. The battery capacity can be preset or learned during the subsequent full charge cycle. This method can achieve satisfactory accuracy after compensating for self-discharge of the battery, capacity change at different temperatures, etc., and is therefore widely used in high-end applications such as notebook computers.
How does the battery fuel gauge work?
The battery fuel gauge continuously integrates the total current flowing into/out of the battery, and uses the net charge amount obtained by the integration as the remaining capacity. 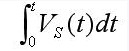
A simplified battery fuel gauge is shown in Figure 1. Among them, RSNS is a mΩ-level current-sense resistor, and RL is a load resistor. The voltage drop generated by the current IO of the battery through the switch and RSNS to the RL discharge is VS(t)=IO(t)×RSNS. The fuel gauge continuously detects the differential pressure VS across the RSNS and converts it to the N-bit digital Current (CR) through the ADC, and then accumulates at the rate determined by the time base. The M-bit accumulation result is the unit of Accumulated_Current (referred to as ACR). It is Vh (volts). Accumulating the quantized VS is equivalent to integrating it, and the result is.
battery power 
The actual fuel gauge also includes some control and interface logic, and usually detects parameters such as battery voltage and temperature. Some smart fuel gauges automatically correct battery self-discharge and save battery characteristics, allowing users to customize battery power calculations. 
Battery fuel gauge calculation
Usually, the unit of CR in the fuel gauge data is mV, and the unit of ACR is mVh.
According to the previous description, the CR value is the voltage value across the sampling resistor. A typical 12-bit CR is shown in Table 2.
Where S is a sign bit and 20 is an LSB. If the full bias value of CR is F, then the LSB is calculated as follows:
(1) 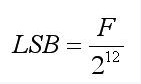
If the CR reading is M and the sampling resistance is the value RSNS, the actual current value is:
(2) 
The direction of the current is determined by the S bit. If the full bias value F is ±64mV, the LSB is ±15.625μV; when RSNS is 10mΩ, the maximum current is ±6.4A. If M is 768, the actual current is 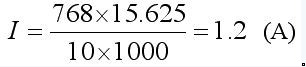 .
.
ACR is the cumulative value of the voltage across the sampling resistor. A typical 16-bit ACR is shown in Table 3.
Where S is a sign bit and 20 is an LSB. If the full-bias value of ACR is F, the formula for calculating LSB is as follows:
(3) 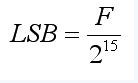
The net charge is determined by the S bit. If the full bias value F is ±204.84mVh, the LSB is ±6.25μVh; when the RSNS is 10mΩ, the maximum power is ±20.48Ah. If M is 7680, the actual power is . 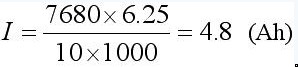
Conclusion
After introducing the principle of the battery fuel gauge, this paper gives some simple calculation formulas. Designers can easily calculate the actual amount of electricity from the fuel gauge readings to speed up the design process.
Currently, a large number of rechargeable batteries include lead-acid batteries , nickel-cadmium/nickel-hydrogen batteries, and lithium ion/lithium polymer batteries. The characteristics of these batteries are shown in Table 1.